Advanced Platform Technology Center
Allison Hess-Dunning, PhD
 |
Research Interests Neural Interfaces, Microfabrication and polymer-based microdevices, Drug delivery, Biosensors, Novel materials for implanted microdevices |
Dr. Hess-Dunning is an Investigator with the APT Center and the Louis Stokes Cleveland VA Medical Center. She is also a Research Assistant Professor in the Department of Biomedical Engineering at Case Western Reserve University. She received a B.S. in Engineering Physics from the University of Pittsburgh, and M.S. and Ph.D. degrees in Electrical Engineering from Case Western Reserve university. Since joining the VA, Dr. Hess-Dunning has received Career Development Awards (CDA-1 and CDA-2) and has served as PI or Co-PI on two Merit Review Awards from the Rehabilitation Research and Development Service. Additionally, Dr. Hess-Dunning is the co-director for the APT Center’s Wen H. Ko and DEI Summer Internship Programs that provide VA research opportunities for undergraduates. Dr. Hess-Dunning’s research efforts focus on developing multi-functional, responsive intracortical interfaces to enable intracortical brain-machine interfaces with long-term reliability. Her research is highly interdisciplinary, involving identifying and/or developing well-suited materials for neural interfaces, development of manufacturing processes to build microscale devices that integrate novel materials, neural interface device design, and pre-clinical studies.
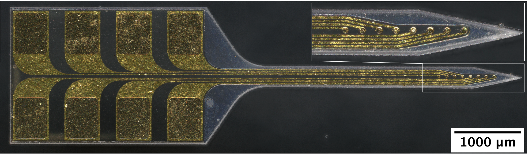
Dr. Hess-Dunning’s team has developed mechanically-adaptive neural probes that can be inserted into tissue while stiff but then rapidly soften after insertion to minimize mechanical mismatch with brain tissue. By building the neural probe from materials that are more similar to brain tissue, we expect to see a reduction in neuroinflammation surrounding the probe and improvements in neural probe integration with tissue. Additionally, anti-inflammatory or anti-oxidative agents can be incorporated into the mechanically-adaptive polymer nanocomposite structural material to further reduce neuroinflammation and oxidative stress within the first weeks after implantation. Therapeutic agent administration can be extended by incorporating microfluidic channels that interface with a drug reservoir and pumping mechanism. The combination of mechanical flexibility and local therapeutic delivery can improve recorded signal quality from nearby neurons. This work can aid in our understanding of neural circuitry and improve the performance of brain-machine interfaces for individuals with neurological injuries or diseases.
Dr. Hess-Dunning can be reached by email at allison.hess@case.edu
  |
|
Figures: (Top) Mechanically-adaptive neural probe for intracortical recording. The polymer nanocomposite structure can be loaded with a therapeutic agent (e.g., resveratrol) during synthesis to treat neuroinflammation or oxidative stress locally. (Bottom) Microfluidic channels integrated into the mechanically-adaptive neural probe for chronic drug delivery applications. The probe interfaces with drug reservoirs and pumps through a custom connector and tubing. |



















